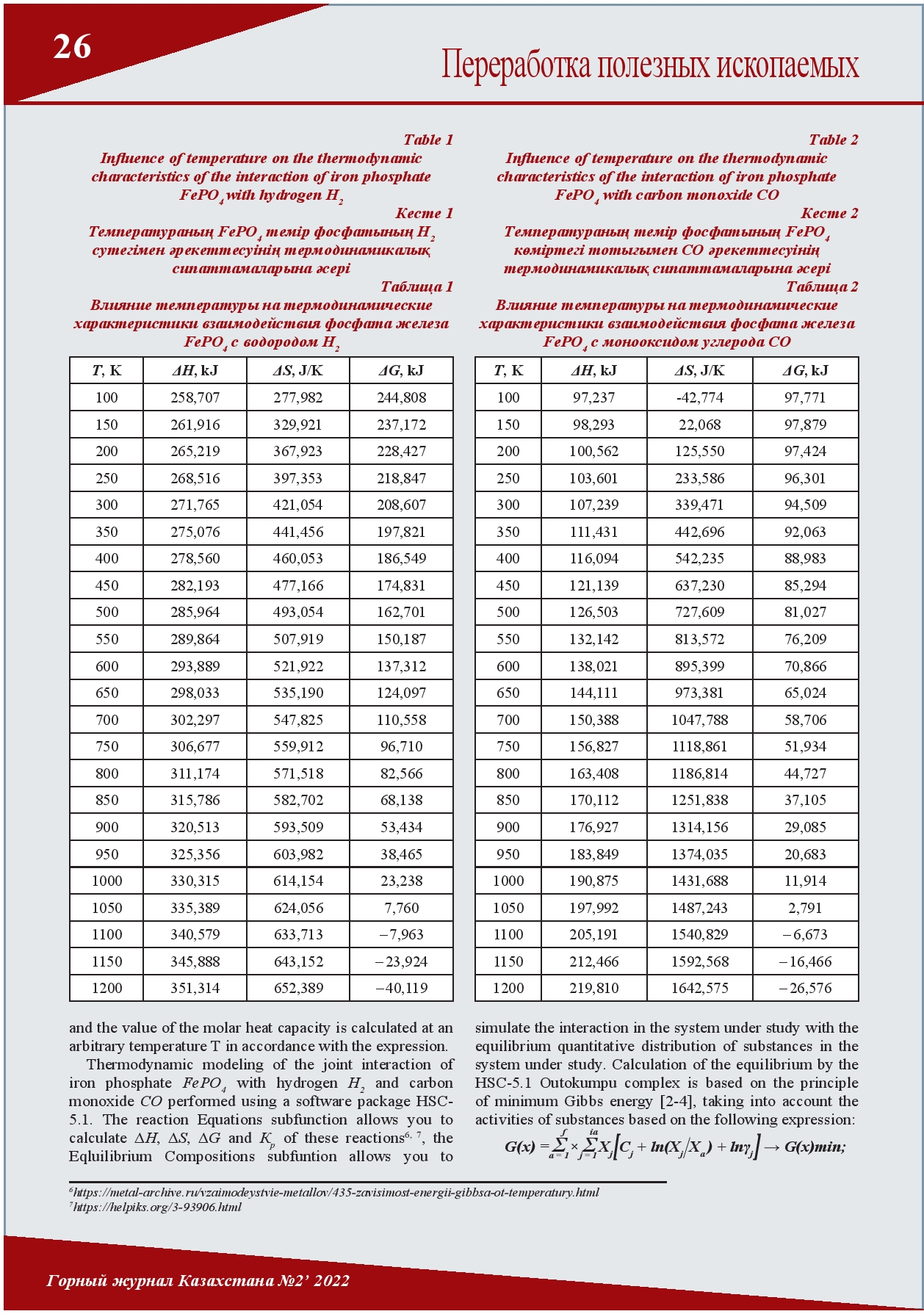
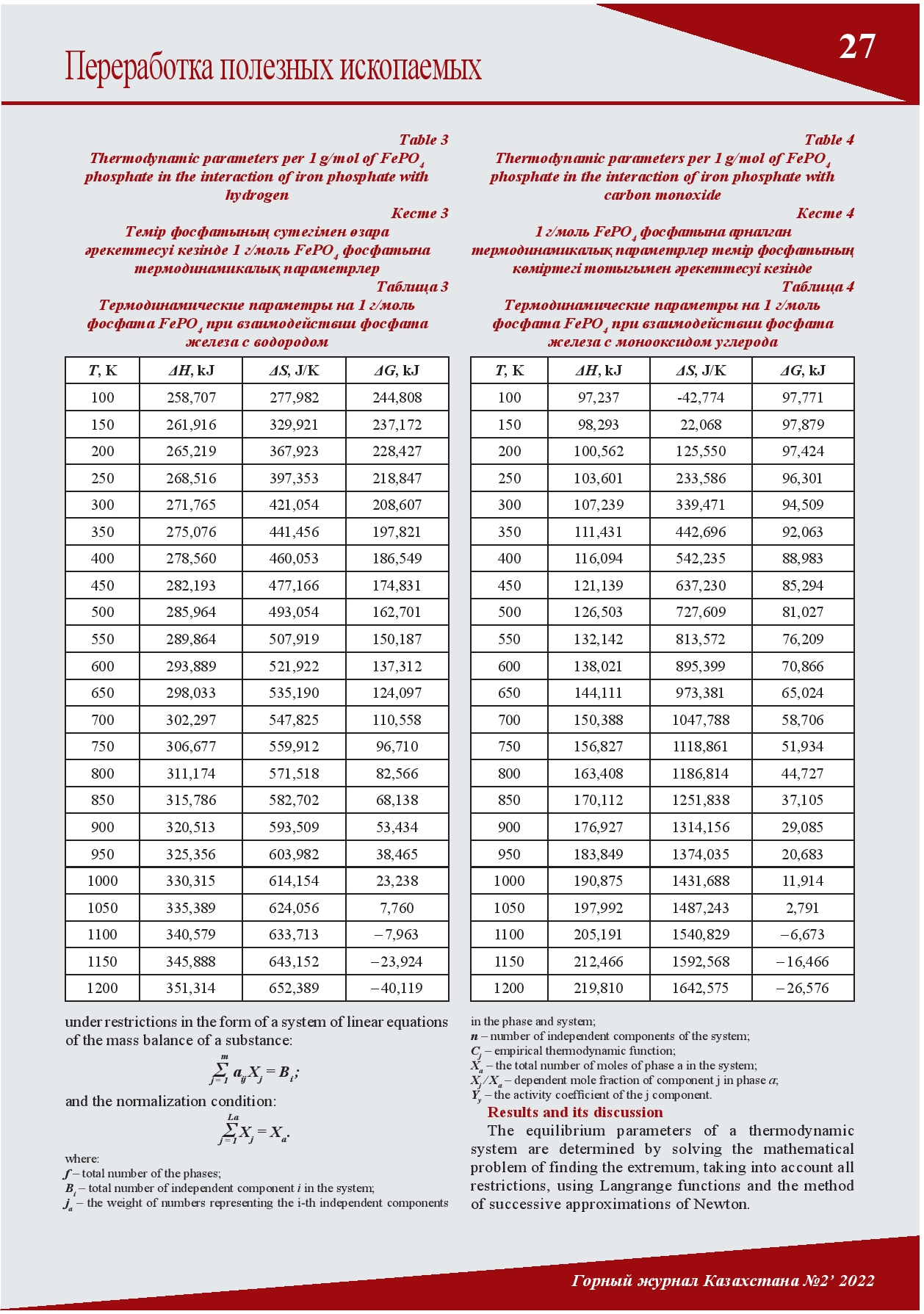
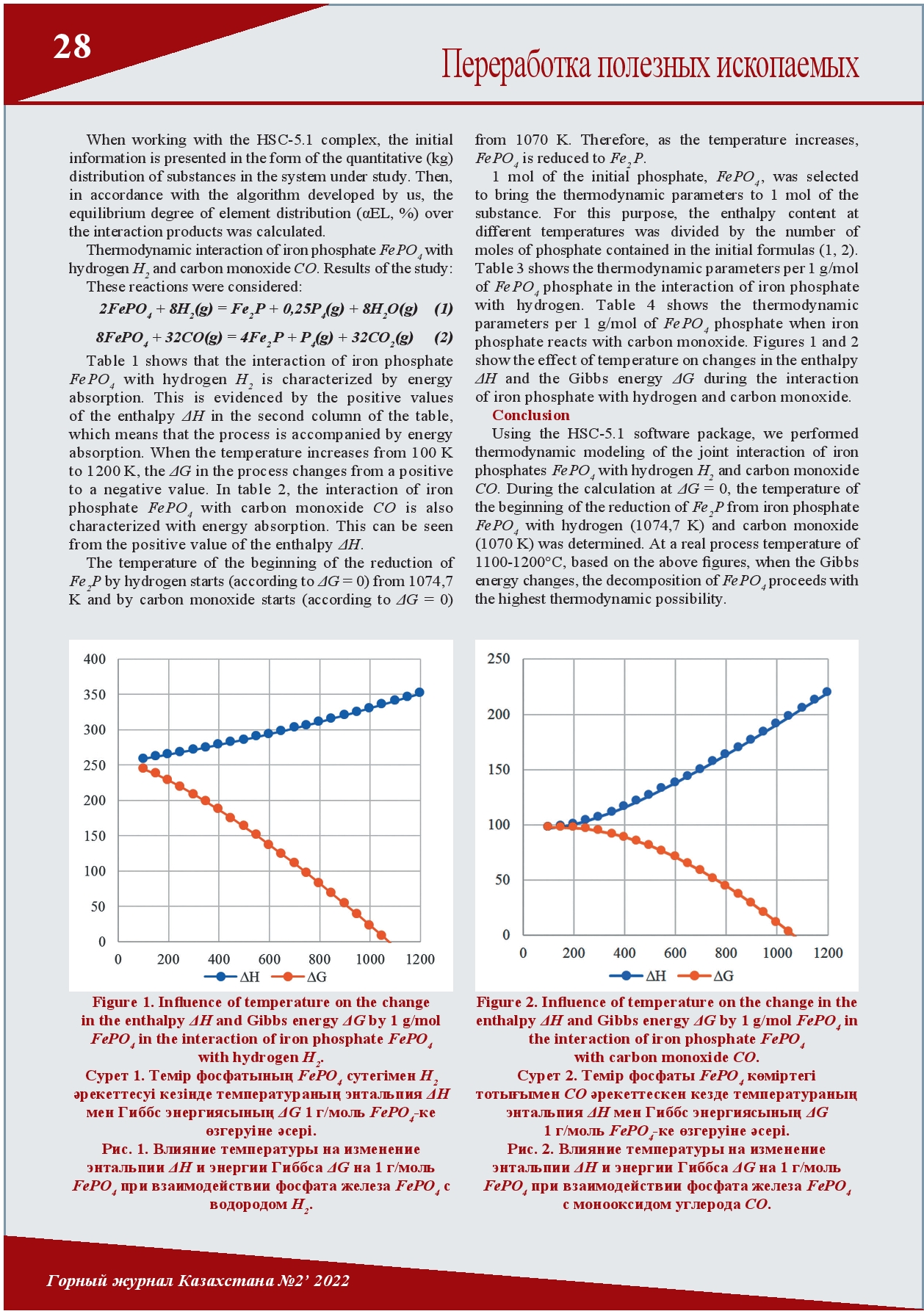
THE INFLUENCE OF TEMPERATURE ON THE CHANGE OF THE GIBBS FREE ENERGY IN THE THERMODYNAMIC INTERACTION OF IRON PHOSPHATE WITH HYDROGEN AND CARBON MONOXIDE
https://doi.org/10.48498/minmag.2022.202.2.003
Today, the Lisakovskoye deposit of oolitic iron ores is one of the raw material bases of the ArcelorMittal Temirtau Joint Stock Company. However, their use in metallurgical production is currently limited due to the high content of phosphorus. It has been established that phosphorus in the Lisakovsky concentrate is in the form of various minerals (oolites, vivianite, hydrogoethite) and compounds (iron phosphates, hydrated phosphorus-containing components). By interacting with gaseous compounds of these various iron phosphates, conditions can be created to reduce excess phosphorus. This article discusses the thermodynamic interaction of iron phosphate with hydrogen and carbon monoxide.
Ye. Mukhametkhan, M. Mukhametkhan, G.G. Zhabalova, V.M. Shevko